Abstract
ABSTRACT
Background. Hodgkin Lymphoma (HL) Hodgkin Lymphoma (HL) is a B-Cell neoplasia characterized by a high degree of response to chemotherapy and an overall favourable outcome in responding patients. Despite the efficacy of first line therapy, however, about 30% of patients affected by HL are either refractory or eventually relapse (R/R) after first or second line therapy followed by autologous hemopoietic stem cell transplantation (ASCT). Immune checkpoint inhibitors (CI) have demonstrated clinical activity in HL patients relapsing after ASCT, although only 20% complete response (CR) rate are usually observed. The efficacy of CI is strictly related to the host immune competence, which is impaired in heavily pre-treated HL patients.
Aims. Here, we aimed to enhance the activity of early post-ASCT CI (nivolumab) administration with the infusion of autologous lymphocytes (ALI) and to investigate the lymphocyte subpopulation involved in the mechanism of response to CI in HL.
Results. Seventeen patients with relapse/refractory (R/R) HL (median age 29 years; range 18-65), underwent lymphocyte apheresis after first line chemotherapy and then proceeded to salvage therapy. Subsequently, 12 patients with progressive disease at ASCT received early post-transplant CI supported with four ALI, whereas 5 responding patients received ALI alone, as a control cohort. Most patients showed CMV seropositivity at the time of enrolment, without signs of active infection (positive anti-CMV IgG, negative IgM and undetectable CMV DNA).
No adverse events were recorded, specifically, no immune-related toxicity was observed and none of the patients showed CMV reactivation. All patients receiving ALI + CI (treated patients) achieved negative PET scan CR and 11/12 are alive and disease-free after a median follow-up of 33 months. The only relapse occured 23 months after last ALI.
Six of the responding patients underwent subsequent allogeneic SCT in a CR status and 1 patients are currently waiting for transplantation. Two of the patients in the control arm relapsed after a median of 6 months (4-8 months)
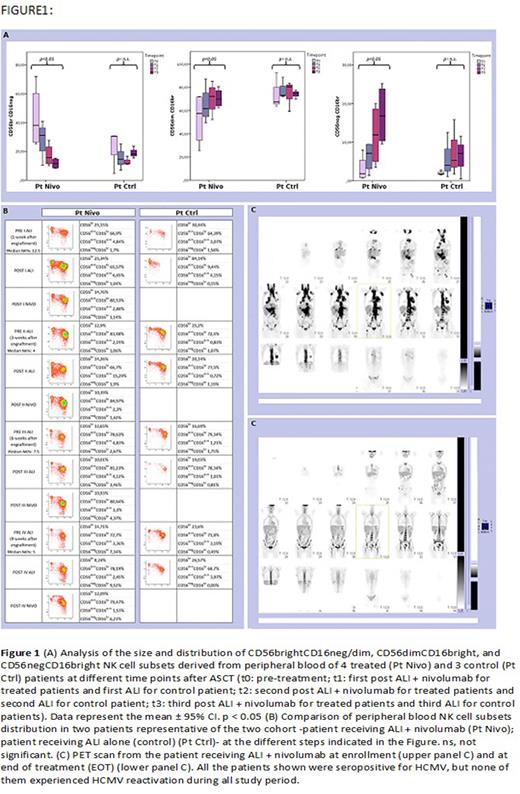
Phenotypic analysis of circulating cells showed a faster expansion of highly differentiated NK cells in ALI plus nivolumab-treated patients as compared to control patients (Fig.1).
In detail, different patterns of NK cell development could be identified. Indeed, starting from one month after transplantation, in the group of patients undergoing ALI plus nivolumab, a relevant fraction of NK cells displayed a mature phenotype characterized by a CD56dimCD16bright expression (Fig. 1). On the contrary, in control patients, NK cells were characterized by a more immature phenotype (high frequencies of CD56brightCD16neg/dim NK cells) even at late time points (three months) after transplantation (Fig. 1). This event occurred in all the treated (ALI + nivolumab) patients analyzed (Fig. 1). Finally, PB from patients undergoing ALI + nivolumab was enriched in unconventional CD56dimCD16dim NK cells (Fig. 1).
One month after transplantation, CD94/NKG2A+ KIR- NK cells were largely predominant in all patients, whereas CD94/NKG2A- KIR+ NK cells were present in low proportions. An increase of CD94/NKG2A- KIR+ subset occurred primarily in treated patients starting from t2. At t3, the significant increase of CD94/NKG2A- KIR+ NK cells occurred in the treated group but not in the control group . This event occurred in all the treated (ALI + nivolumab) patients analyzed, regardless of their serological status for HCMV, but was more evident in treated patients who were seropositive for HCMV.
Remarkably, PB from HCMV seropositive patients undergoing ALI + nivolumab became enriched over time in differentiated NK cells characterized by high expression of NKG2C receptor (the activating counterpart of NKG2A), as compared to HCMV+ control patients. This subset represents the so-called "adaptive" NK cell population that usually increases during HCMV infection/reactivation. In this case, however, this event occurred in the absence of HCMV reactivation, and only in HCMV seropositive patients treated with ALI + nivolumab.
Conclusions. Our data show anti-tumour activity with good tolerability of ALI + CI for R/R HL and suggest that this setting may accelerate NK cell development/maturation and favour the expansion of the "adaptive" NK cell compartment, especially in patients with CMV seropositivity, in the absence of CMV reactivation.
Disclosures
Agostini:Hemanext: Research Funding. Lemoli:Stemline Therapeutics: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Celgene: Research Funding; Novartis: Consultancy; Jazz: Consultancy; Daiichi Sankyo: Consultancy; Servier: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.